Ionic compounds polyatomic ions worksheet answers – Embark on a journey into the fascinating world of ionic compounds and polyatomic ions with our comprehensive worksheet answers. This guide will unravel the intricacies of these chemical entities, providing a deep understanding of their properties, reactions, and applications.
Delving into the realm of ionic compounds, we will explore their formation, naming conventions, and the unique characteristics that distinguish them from other compounds. We will also delve into the fascinating world of polyatomic ions, uncovering their structures, charges, and the role they play in shaping the properties of ionic compounds.
Ionic Compounds and Polyatomic Ions
Ionic compounds are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Polyatomic ions are ions that are composed of more than one atom. They typically have a negative charge and can be either cations or anions.
Some common ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium oxide (CaO). Some common polyatomic ions include the hydroxide ion (OH –), the sulfate ion (SO 42-), and the nitrate ion (NO 3–).
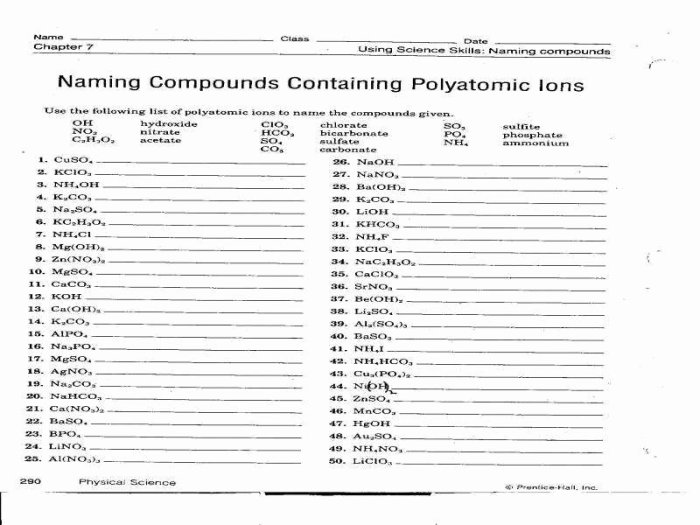
When naming ionic compounds containing polyatomic ions, the name of the cation is followed by the name of the polyatomic ion. For example, sodium hydroxide is named by combining the name of the sodium cation (Na +) with the name of the hydroxide ion (OH –).
Properties of Ionic Compounds
Ionic compounds typically have high melting and boiling points. They are also good conductors of electricity when dissolved in water or melted. The physical and chemical properties of ionic compounds are influenced by the size and charge of the ions.
Smaller ions tend to form stronger bonds and have higher melting and boiling points. Ions with higher charges also tend to form stronger bonds.
Ionic compounds are generally soluble in water. The solubility of an ionic compound depends on the size and charge of the ions. Smaller ions tend to be more soluble than larger ions. Ions with higher charges also tend to be more soluble than ions with lower charges.
Reactions of Ionic Compounds: Ionic Compounds Polyatomic Ions Worksheet Answers
Ionic compounds can undergo a variety of reactions, including precipitation reactions and acid-base reactions.
Precipitation Reactions, Ionic compounds polyatomic ions worksheet answers
A precipitation reaction is a reaction in which two ionic compounds are mixed together and a solid precipitate forms. For example, when sodium chloride (NaCl) is mixed with silver nitrate (AgNO 3), a white precipitate of silver chloride (AgCl) forms.
Acid-Base Reactions
An acid-base reaction is a reaction in which an acid and a base react to form a salt and water. For example, when hydrochloric acid (HCl) is mixed with sodium hydroxide (NaOH), sodium chloride (NaCl) and water (H 2O) are formed.
Applications of Ionic Compounds

Ionic compounds have a wide variety of applications in everyday life. They are used in everything from fertilizers to food additives to medicines.
- Fertilizers: Ionic compounds are used as fertilizers to provide plants with the nutrients they need to grow. For example, ammonium nitrate (NH 4NO 3) is a common nitrogen fertilizer.
- Food additives: Ionic compounds are used as food additives to improve the taste, appearance, or shelf life of food. For example, sodium chloride (NaCl) is used as a flavor enhancer and preservative.
- Medicines: Ionic compounds are used in a variety of medicines to treat a variety of diseases. For example, sodium bicarbonate (NaHCO 3) is used as an antacid to treat heartburn.
Ionic compounds also play an important role in biological systems. For example, sodium ions (Na +) and potassium ions (K +) are essential for the proper functioning of nerves and muscles. Calcium ions (Ca 2+) are essential for the formation of bones and teeth.
Ionic compounds are also used in a variety of industrial applications. For example, sodium chloride (NaCl) is used in the production of chlorine and sodium hydroxide. Calcium carbonate (CaCO 3) is used in the production of cement.
Popular Questions
What is the difference between an ionic compound and a polyatomic ion?
Ionic compounds are formed by the electrostatic attraction between positively charged metal ions and negatively charged non-metal ions. Polyatomic ions are groups of atoms that carry a net electrical charge and behave as a single unit within an ionic compound.
How do you name ionic compounds containing polyatomic ions?
To name ionic compounds containing polyatomic ions, the name of the metal ion is followed by the name of the polyatomic ion, with the suffix “-ide” added to the root of the polyatomic ion’s name.
What are some common applications of ionic compounds?
Ionic compounds have a wide range of applications, including their use as fertilizers, pigments, food additives, and in the production of batteries, glass, and ceramics.